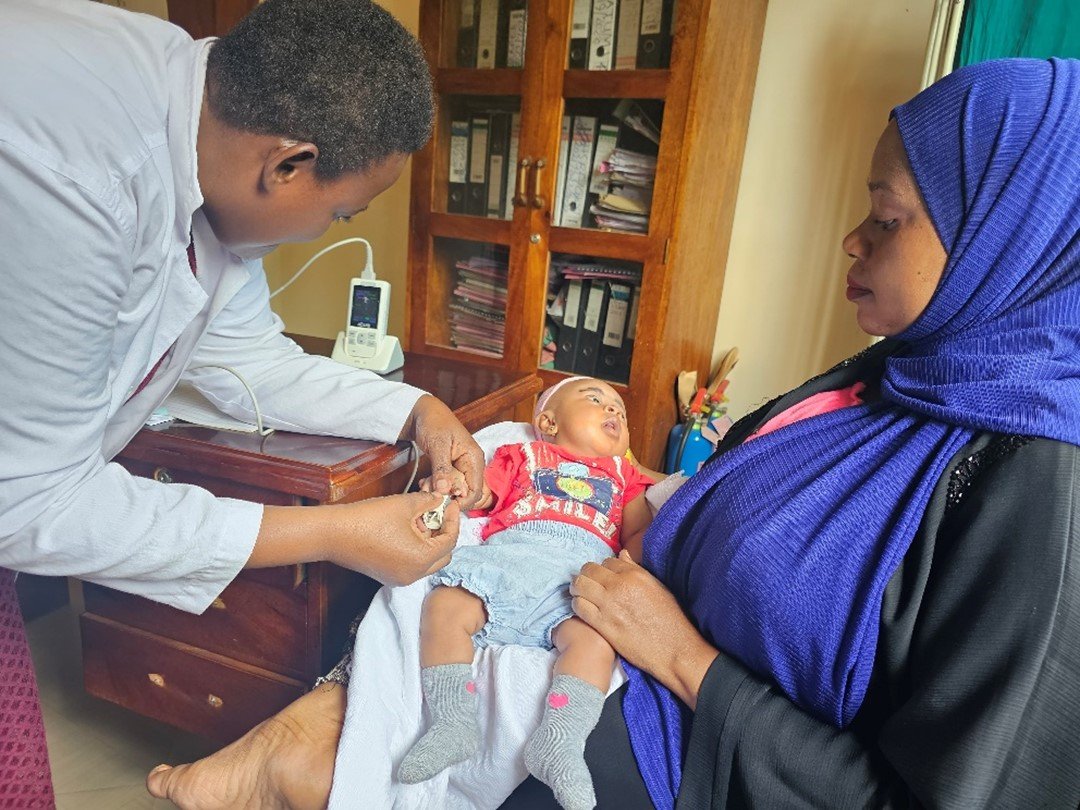
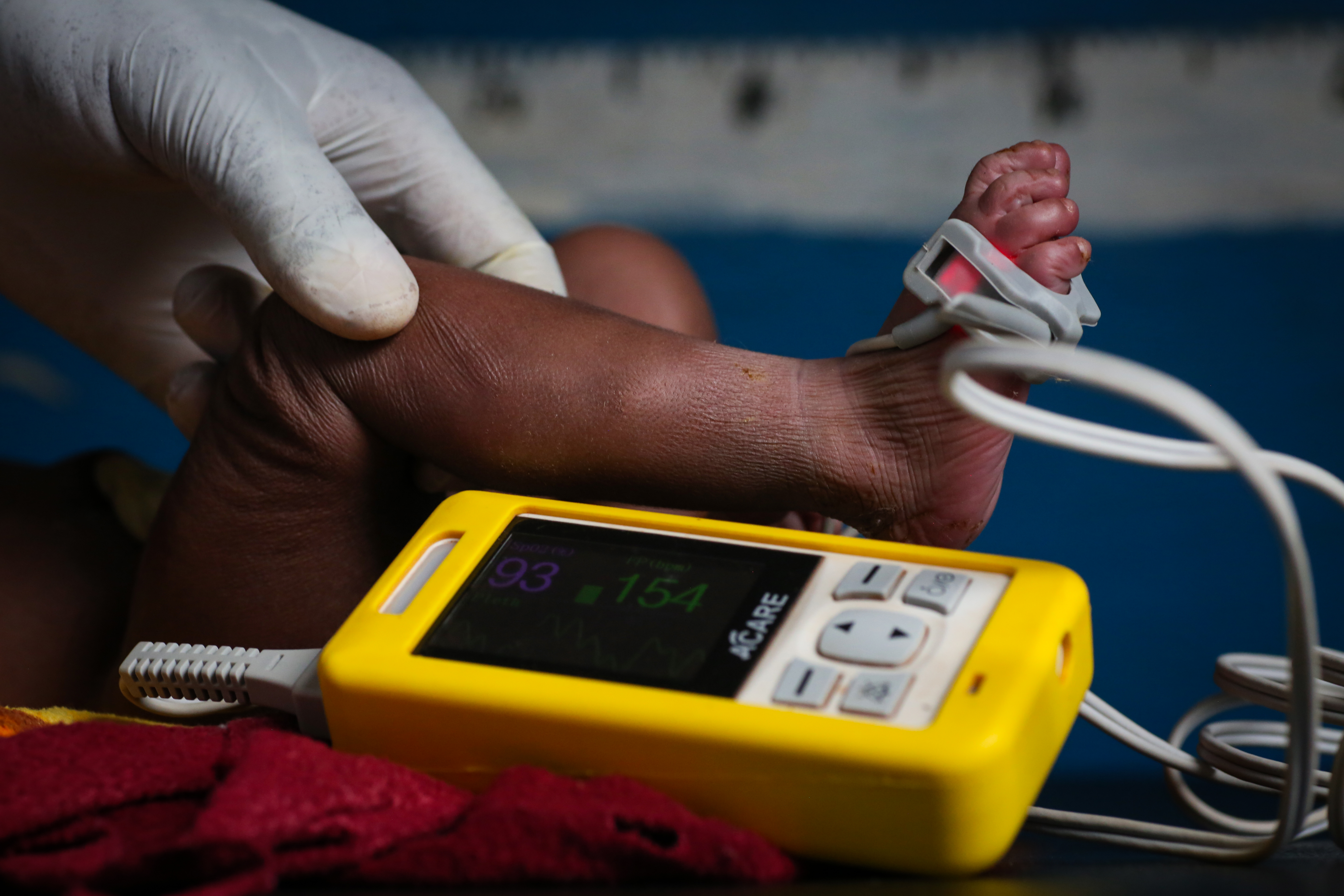
Protects the most vulnerable
Prevents malaria
Reduces hospitalizations
Complementary tool
What is the RTS,S vaccine?
RTS,S is the world’s first malaria vaccine to show significant efficacy in preventing malaria in young children. Developed over several decades, RTS,S targets Plasmodium falciparum, the deadliest malaria parasite, and is designed for use in regions with high transmission rates. The World Health Organization recommended the RTS,S vaccine based on data gathered through malaria vaccine pilots in Kenya, Ghana and Malawi, co-funded by Unitaid, the Global Fund and Gavi.
How effective is the vaccine?
A Phase 3 clinical trial found that among children aged 5-17 months who received four doses of RTS,S, the vaccine prevented 39% of malaria cases over four years of follow up, and 29% of severe malaria. Significant reductions were also seen in overall hospital admissions as well as in admissions due to malaria or severe anemia. In addition, the vaccine reduced the need for blood transfusions, which are required to treat life-threatening malarial anemia, by 29%.
How could the RTS,S vaccine accelerate access to new malaria vaccines?
The introduction and scale up of RTS,S set a precedent for deploying malaria vaccines in endemic regions. It creates a framework for potential future vaccine development, including newer, more effective malaria vaccines currently in the pipeline. Lessons learned from RTS,S in terms of distribution, acceptance, and integration into national immunization programs can help streamline the rollout of subsequent malaria vaccines, potentially accelerating their access.
What role did Unitaid play in scaling up RTS,S?
With our partners, our support to the malaria vaccine implementation program enabled large-scale pilot programs to evaluate vaccine impact, develop implementation models and address barriers to widespread adoption, working in collaboration with our partners. The evidence gathered through these pilot programs underpinned the WHO recommendation of RTS,S in 2021.