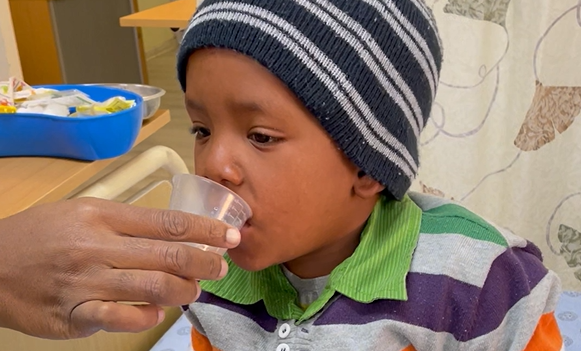
The problem
Multidrug-resistant tuberculosis (MDR-TB) is a strain of TB that does not respond to the primary drugs used to treat it. Every year, an estimated 30,000 children develop MDR-TB, yet fewer than 15% of them are diagnosed and start treatment. For those who do, regimens are long, bad tasting and toxic, and can cause severe side effects such as irreversible hearing loss. Medicines were not designed for administration to children, requiring caregivers to cut, break, crush and mix many bad-tasting adult tablets to treat children. Low demand, lack of availability of child-friendly options and weak data on new treatments limited access to better medications.
The complexity, burden and cost of treating children with MDR-TB makes preventive regimens even more critical. However, a lack of strong data about preventive medications kept children from receiving them, although in most cases these medications could stop the disease from developing.
Our response
The BENEFIT Kids project aimed to improve MDR-TB treatment and prevention options for children of all ages with child-friendly formulations and preventive therapy.
The project led a clinical trial investigating MDR-TB preventive regimens for children that proved to cut a child’s risk of developing active disease by more than half. This critical research, presented at the same time as the first-ever study into MDR-TB prevention for adults, underpinned the World Health Organization’s recommendation in early 2024. The project worked to develop a properly dosed, palatable, and easy-to-swallow formulation of the preventive medicine in parallel, meaning the key MDR-TB components were available for purchase even before the recommendation came through.
In addition, the project developed ways to improve the taste and adapt the formulations of all drug components in MDR-TB treatment regimens, and strengthened data on dosing, safety, efficacy, acceptability and costs – a critical step for creating international policies that incentivize the use of these medicines. The project also contributed to shaping markets for these child-friendly formulations.