Amazing progress has been made in the fight against HIV and AIDS. Nearly 30 million people with HIV are on lifesaving antiretroviral therapy (ART) – a fourfold increase from 2010 – and 82% of pregnant and breastfeeding women are receiving medication to improve their own health and to prevent transmission of HIV to their babies.
But challenges remain on the path to end HIV and AIDS as a global health threat. Out of the nearly 39 million people living with HIV worldwide, despite the reach of current services, 9.2% are not accessing treatment and 14% of people with HIV are unaware of their status. In the absence of sustained treatment, people living with HIV can continue to transmit the virus to others.
Adolescent girls and young women are disproportionately affected. Every week, more than 4,000 adolescent girls and young women are newly infected; in sub-Saharan Africa they are three times more likely than their male peers to contract the virus. Key populations such as gay men and other men who have sex with men, sex workers and their clients, people who inject drugs and transgender people need access to treatment and better tools to protect themselves. Children are being left behind; only half of children receive timely HIV diagnosis and are on ART, compared with three-quarters of adults. More than 130,000 children were newly infected with the virus in 2022 and 84,000 children died.
How we work
At Unitaid, we save lives by making new health products available, adapted, and affordable for people in low- and middle-income countries. We identify challenges that are slowing progress towards global health goals, find and invest in innovative products and solutions, then work with countries and partners to take them to scale so people everywhere can benefit.
We work with a broad range of partners – researchers, manufacturers, governments, donors, regional and global health partners and affected communities – to identify, invest in, and then take to scale promising new technologies and strategies for prevention, diagnosis and treatment for HIV and coinfections. Through our unique market-shaping role, we have helped introduce groundbreaking, affordable HIV tests, treatments and prevention tools, including for coinfections and comorbidities, that are used as standard of care in more than 110 countries worldwide.
Key HIV health products introduced by Unitaid:
- All first-line medicines for HIV in Africa, including dolutegravir, the most effective and affordable treatment to date that will save countries and partners US$8 billion by 2030
- 1st HIV treatments and quality-assured diagnostics for children, now used in 100+ countries
- HIV self-tests, a critical tool to increase diagnosis and treatment of people with HIV
- The full range of pre-exposure prophylaxis (PrEP) medicines to prevent HIV, including oral PrEP, long-acting injectable cabotegravir and the dapivirine vaginal ring
- Key tools for the advanced HIV’s care package including the first CD4 rapid diagnostic test, screening tools and medicines for leading deadly coinfections such as TB and cryptococcal meningitis
- Key tools for management of other coinfections including cervical cancer and hepatitis
Prevention
While deaths from AIDS-related illnesses have been declining steadily since the peak of the epidemic, more than 1.3 million people were newly infected in 2022 and new infections are rising among certain populations. To protect people from contracting HIV, Unitaid prioritizes expanding access to a range of effective prevention strategies.
We contributed to the scale-up of oral PrEP and are supporting the introduction of new long-acting PrEP products to enable users to choose the method that best suits their needs and preferences, particularly for high-risk and key populations such as adolescent girls and young women, gay men and other men who have sex with men, and transgender people.
We invest in initiatives that remove barriers to accessing PrEP and integrate PrEP into comprehensive sexual and reproductive health services, including for sexually transmitted infections (STIs), with a particular focus on adolescents. We work with the Medicines Patent Pool (MPP), an organization founded by Unitaid in 2010, and generic manufacturers to ensure affordability and widespread availability of all PrEP choices. For example, we supported MPP to secure the generic license for long-acting cabotegravir, the first injectable form of PrEP, and support generic manufacturers with product development and regulatory strategies. We also work to ensure equitable access is planned from the earliest stages for products in the pipeline, such as lenacapavir.
We support programs and studies that catalyze uptake of intervention packages to eliminate vertical transmission (from mother to child) of HIV, syphilis, hepatitis B and Chagas disease, and to increase access to ART for pregnant and breastfeeding women to protect their health and ensure their babies are HIV-free. We invest in programs that provide harm reduction services, such as clean needles and syringes and long-acting opioid substitution therapy, to prevent transmission of HIV and Hepatitis C among people who inject drugs.
Testing
Early diagnosis is critical to ensure people access lifesaving treatment and to prevent onward transmission, yet nearly 6 million people living with HIV are unaware of their status. Fear of stigma and discrimination and challenges accessing testing remain key factors behind low testing rates in many communities.
We support the development, pre-qualification and market introduction of affordable, quality diagnostics for HIV. This includes self-testing kits that people can use in the privacy of their own homes, along with promoting awareness and education to encourage their use. We invested in accurate point-of-care diagnostic tests that deliver results within minutes at the community level; many of these devices have multidisease capabilities, including for coinfections such as hepatitis C, tuberculosis (TB), human papillomavirus (HPV, which can cause cervical cancer) and other STIs. Together with FIND, we’re investing in regional manufacturing of HIV rapid diagnostic tests, which helps strengthen local markets for HIV health products, strengthening access, sustainability of supply and health security.
Treatment
We work to ensure access to effective and affordable treatments for all people living with HIV. We helped introduce and lower prices for ARV combination regimens, including dolutegravir, the standard of care now used by more than 24 million people in low- and middle-income countries and available at less than US$45 a year – the lowest price ever for an ART. We helped introduce the first-ever child-specific ART in 2006 and since then have supported development, access and introduction of multiple child-friendly formulations and are driving the push to increase coverage for all children with HIV. We are supporting the development of long-acting injectable ARTs, enhancing adherence and health outcomes.
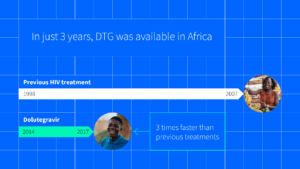
Case study: Delivering a best-in-class HIV drug – three years faster
When the innovative new HIV drug dolutegravir (DTG) was approved in 2014 – faster working, with fewer side effects, less prone to resistance and with the potential to be less expensive than the existing first-line ART at the time – Unitaid brought together partners, including the Medicines Patent Pool, manufacturers, researchers, communities and scale-up partners like the Global Fund to make DTG affordable and accessible.
We partnered with research institutions to generate evidence for use of dolutegravir in different sub-populations, including people living with HIV in sub-Saharan Africa, neonates and pregnant women, and supported market interventions and worked with communities for introducing and scaling up the new DTG regimen. In just three years, DTG was introduced in low- and middle-income countries – three times faster than previous regimens.
Prevention, testing and treatment for coinfections
People with HIV, if they are not taking ART to control the virus, are at risk of contracting coinfections, which can quickly progress to serious illness or death. We enhance access to critical tools for testing, preventing and treating opportunistic infections such as TB and cryptococcal meningitis, which are prevalent in people with advanced HIV disease.
Tuberculosis: TB is the main cause of death among people living with HIV, accounting for one-third of AIDS-related deaths worldwide. We invest in programs that provide comprehensive care and treatment for HIV and coinfections within a single health care delivery system, including testing for both diseases at once, reducing the burden on patients and improving health outcomes. We support research and development of new diagnostic tools and treatment regimens specifically tailored for coinfections in people living with HIV. We also introduced the first-ever child formulation of TB medication.
Hepatitis C: We are supporting elimination of hepatitis C through prevention, testing and treatment. We have helped make treatment more affordable, introduced simpler and better tests, including rapid diagnostic tests and self-tests, and simplified the approach to testing and treatment. We are working to introduce innovative prevention tools and supporting long-acting technologies with the aim to develop a treatment that could cure hepatitis C with a single injection.
Cervical cancer: Women with HIV are six times more likely to develop cervical cancer and 9 in 10 women who die from it live in low- and middle-income countries. To find and treat women with cervical cancer, we’ve worked to establish a market for HPV testing – the virus that can cause cervical cancer – with HPV self-collection tools that women can use in the privacy of their homes, cervical cancer thermal ablation devices, and point-of-care screening and treatment for other STIs. We’ve also worked with countries to establish models of delivery, including integrated models of care across HIV and cervical cancer.
Looking ahead
While HIV is no longer the death sentence that it once was, we cannot lose momentum. New treatments, tests and prevention tools are important parts of the solution, but we need to rapidly increase access for key and vulnerable populations that are often underserved. HIV drug resistance, a form of antimicrobial resistance where pathogens develop resistance to the drugs used to treat them, is a growing threat.
Through our innovative strategies, focus on market access, and commitment to empowering communities, Unitaid is accelerating progress towards the global goal of ending HIV as a global health threat. We can’t stop until everyone, everywhere – particularly vulnerable groups like adolescent girls and young women, children, and key populations – have access to affordable, effective and fit-for-purpose prevention tools, tests, treatments and care.
Additional resources:
Related news:
- New Unitaid report: World’s leading HIV drug proven to have reduced global CO2 emissions by 26 million tons
- Unitaid calls for accelerated global access to long-acting HIV prevention drug lenacapavir after positive trial results
- World AIDS Day: Let Communities Lead!
- Global Fund, PEPFAR and Unitaid Collaboration Paves Way to Accelerate Approval of African-Manufactured HIV Rapid Tests
