The problem
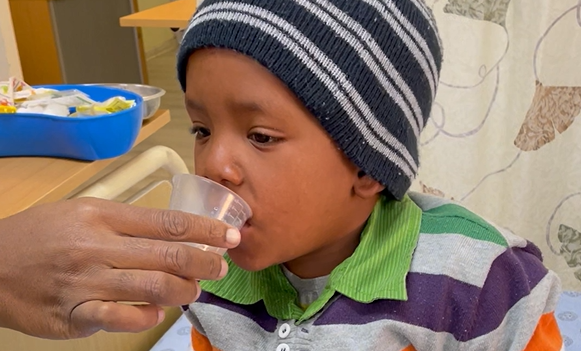
In 2015, an estimated one million children became ill with tuberculosis (TB) but treating children was complex because child-adapted medicines weren’t available. Children need different medication dosages than adults, and flavors and formulations to make the medicines easier to swallow. Without this, caregivers had to break tablets meant for adults into smaller pieces to administer to children, who often refused to swallow them because of the bitter taste. This could result in incorrect dosing, incomplete treatment, and poor outcomes.
Download the project evaluation
Our response
In December 2015, the STEP-TB project launched the first fruit-flavored, affordable, appropriately formulated, first-line pediatric TB medicines in high-burden countries in both the public and private sectors. The project also contributed to better estimates of the burden of childhood TB, which is important information for medicine manufacturers. As of 2024, child-friendly TB medicines were available in 123 countries.