Failure to implement contact tracing and tuberculosis prevention would result in close to 1 million deaths by 2035, according to new study
Combination intervention found to be cost-effective in averting illness and deaths in high-risk groups
- People living in close contact with a person with TB disease are at highest risk of infection, and account for a significant percentage of the 10.6 million new TB infections each year.
- Analysis shows that implementing a combined strategy of identifying household contacts and providing TB preventive treatment is cost-effective and would cut deaths by 35% among household contacts of all ages and people living with HIV by 2035.
- Additionally, because TB diagnosis is so low among children under five – just over 3 in 10 children with TB are identified – contact tracing and prevention would have an outsized impact on reducing child death from TB.
- TB prevention and contact tracing can be delivered cost effectively thanks, in part, to the significant price reductions in short-course therapy achieved in recent years. With further decreases in price and by improving the efficiency and integration of contact tracing into disease responses, the intervention could benefit from greater cost savings and public health benefit.
- As world leaders prepare for the second United Nations High-Level Meeting on TB this September, up-front multi-stakeholder commitment and financial backing is urgently needed to reap the massive rewards of preventing TB illness and death.
Johannesburg/Geneva – A new study published today in The Lancet Global Health found that the lives of 850,000 people could be saved by 2035 if short-course tuberculosis (TB) preventive treatment is provided to people living with HIV and contacts of individuals newly diagnosed with TB. 700,000 of those lives saved would be among children aged 15 years and younger.
The study, co-authored by researchers from Johns Hopkins University, the Aurum Institute and global health agency Unitaid, also found the combined intervention of contact tracing and TB prevention to be broadly cost-effective for household contacts of all ages. The impact was particularly high among children under the age of five who face higher risks of death.
“Tuberculosis remains the world’s deadliest infectious disease, despite being preventable and curable,” said Professor Gavin Churchyard, Group Chief Executive Officer of the Aurum Institute. “Although progress has been made in preventing TB among people living with HIV, we’ve lagged behind in keeping family members—especially children—free of the disease when a parent becomes sick. This new study, we hope, provides the evidence needed to massively scale up the use of TB preventive treatment among those individuals at risk of developing TB.”
TB preventive therapy has made enormous strides in recent years: new shorter treatment regimens can clear TB infection before it develops into active disease with a once-weekly treatment over twelve weeks, called 3HP, or a daily treatment over one month, called 1HP. And a series of negotiations led by Unitaid, the Aurum Institute, and partners have reduced the price of treatment by more than 70% since 2017.
About one-quarter of the world’s population is infected with TB and at risk of developing active disease, which causes severe illness. The World Health Organization recommends TB preventive treatment for those at highest risk of infection, including people living with HIV and household contacts of people with TB. The first United Nations High-Level Meeting in 2018 set targets to accelerate efforts to end TB as a global health threat, including a goal to reach at least 30 million people with TB preventive treatment.
Only the target to reach people living with HIV was attained, and a corresponding decrease in the number of TB cases and deaths occurred within this population during the COVID-19 pandemic. In contrast, TB cases and deaths increased in all other populations over the same period.
“The imperative for TB prevention is clear,” said Vincent Bretin, Director of Results at Unitaid. “This cost-effectiveness analysis proves that preemptively reaching all at-risk individuals – even when it requires the logistical hurdles of going into communities to find those who may not be actively seeking care – is not just ethically sound. It is a smart investment capable of making an enormous impact on the fight to end TB worldwide.”
The study found that providing 3HP through contact tracing, in which the household members of a person diagnosed with TB are identified, assessed, and treated, could yield an estimated 13% cumulative reduction in the number of contacts developing TB through 2035 and an estimated 35% cumulative reduction in deaths.
Among children under five, the combination of 3HP with contact tracing showed to have a profound impact on the child TB burden overall, helping to drive up the identification of active disease in addition to preventing new infections. The impact on missing cases among this population was so substantial that the intervention would save more lives by treating TB disease and infection than the number of new infections prevented.
The study is the first to provide comparable evidence on the cost-effectiveness of short-course TB preventive treatment for people living with HIV and household contacts in three age groups (< 5, 5-14, and ≥ 15 years old), using consistent methods for all four populations. This provides a clear justification to support policy change and implementation of the life-saving approach where coverage is lagging.
“At the moment, too many family members of people diagnosed with TB are slipping through the cracks and too many lives are being lost,” said Tess Ryckman, faculty member at Johns Hopkins University and lead author of the study. “To finally make a significant dent in the TB epidemic, we need stronger recommendations in favor of TB prevention for household contacts along with a significant boost in resources. The stakes are too high not to act now.”
Despite the cost-effectiveness of TB preventive treatment, the researchers note that the absolute cost of scale up will be substantial. External funding—with an explicit plan to bridge to domestic support as TB burden declines—will be needed, as well as a further decrease in the price of the drug rifapentine, the key cost driver in short TB prevention regimens.
NOTES FOR EDITORS:
The analysis is based on modelling of 29 countries representing a range of income levels, geographic regions, and HIV and TB incidence expected to be applicable to other countries with high burdens of TB.
Burundi, Bangladesh, Brazil, Congo DRC, Ethiopia, Ghana, Haiti, Indonesia, India, Kenya, Cambodia, Liberia, Lesotho, Mongolia, Mozambique, Malawi, Namibia, Pakistan, Rwanda, Somalia, Eswatini, Thailand, Tajikistan, Timor-Leste, Tanzania, Uganda, South Africa, Zambia, Zimbabwe.
Information on the Unitaid-funded IMPAACT4TB Project
The IMPAACT4TB project has been working towards delivering transformational change in the accessibility and use of TB preventive treatment since 2018. Funded by Unitaid and led by the Aurum Institute with a consortium of partners, the project seeks to establish 3HP and 1HP as affordable, quality-assured, less toxic therapies suitable for wide introduction in countries most affected by TB.
Since its inception, IMPAACT4TB has:
- Reduced the price of 3HP from US$72 in 2017 to US$14.25 today
- Increased procurement of 3HP from 35,000 patient courses in 2017 to more than 4 million in 2022
- Improved supply security by bringing two new generic manufacturers into the market
- Helped surpass UN HLM prevention targets for people living with HIV
- Generated the missing evidence to enable people with HIV on first-line treatments to safely take short-course TB preventive treatment
By June 2022, 78 countries were using new short-course TB preventive treatments.
Figures on TB preventive treatment
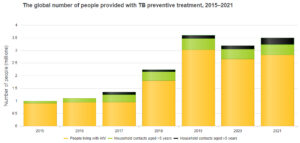
According to the World Health Organization’s 2022 Global TB Report, the total number of people living with HIV and household contacts of people diagnosed with TB who were provided with TB preventive treatment increased from 1 million in 2015 to 3.6 million in 2019. There was a significant decrease in 2020 to 3.2 million, probably reflecting disruptions to health services caused by the COVID-19 pandemic.
The majority of all groups receiving TB preventive treatment are people living with HIV. TB preventive treatment among household contacts continues to represent a significant gap.
Contacts for the media
Kanya Ndaki, KNdaki@auruminstitute.org, +27 83 298 6100
Hervé Verhoosel, verhooselh@unitaid.who.int, +33 6 22 59 73 54
About Unitaid
Unitaid is a global health agency engaged in finding innovative solutions to prevent, diagnose and treat diseases more quickly, cheaply and effectively, in low- and middle-income countries. Its work includes funding initiatives to address major diseases such as HIV/AIDS, malaria and tuberculosis, as well as HIV co-infections and co-morbidities such as cervical cancer and hepatitis C, and cross-cutting areas, such as fever management. Unitaid is hosted by the World Health Organization.
About the Aurum Institute
The Aurum Institute is a proudly African organisation working to advance health science and innovation to create a healthier world for future generations. We partner with governments, the private sector and civil society to design and deliver high-quality care and treatment to people in developing communities. https://www.auruminstitute.org



