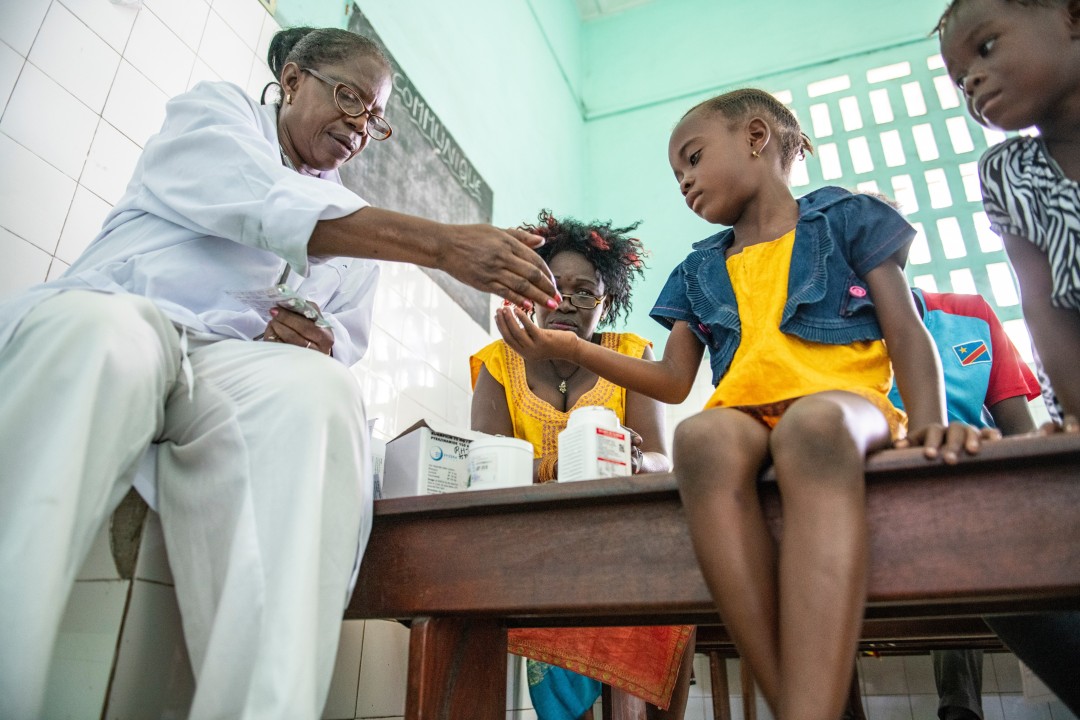The problem
About one-quarter of the world’s population has TB infection, meaning they are infected with the TB bacteria but have no symptoms and cannot transmit the disease. Without treatment, 5% to 10% of those infected will develop active TB, which causes severe illness and can spread from person to person through the air. TB preventive treatment regimens lower the risk of progression to TB after exposure and are particularly vital for children or people with lower immune responses such as pregnant women or people living with HIV or other common coinfections.
Previously, TB preventive treatments lasted anywhere from 6 to 36 months and could cause difficult side effects, which greatly restricted access. Newer, shortened preventive treatment regimens lasting one or three months, called 1HP and 3HP, respectively, could help make treatment possible. Still, with a high pill burden, no child formulation, the high price of treatment, and a lack of evidence to guide implementation and use amongst people on first-line HIV drugs, the new regimens had limited uptake.
Our response
Through the IMPAACT4TB Project, we have expanded access to short-course TB preventive treatment for people living with HIV, children under five years old, pregnant women and household contacts of TB patients. The project seeks to establish these newer regimens as affordable, quality-assured, less-toxic therapies suitable for wide introduction in countries most affected by TB.
Since it began, IMPAACT4TB has reduced the cost of 3HP by 80%, and driven rapid uptake of the preventive treatment, with more than 11 million patient courses purchased across 101 countries by 2024. The project conducted valuable research to ensure young children and people on first-line HIV medicines, including pregnant women, could safely take 3HP or 1HP, helping ensure the populations at highest risk of TB could benefit from the shortened treatments. A child-friendly formulation, available for prices as low or lower than the adult medicines, is now available and the project is working to quickly increase coverage through a seven-country donation program that aims to kickstart demand and broader scale up.
Shorter TB prevention regimens (3HP/1HP)
Medicines that clear TB infection from a person’s system before it develops into active disease.
Our partners
The Aurum Institute leads a consortium of six dedicated partners that have strong experience in shaping markets, strengthening supply chain management and supporting National TB Programs in high burden countries.