The problem
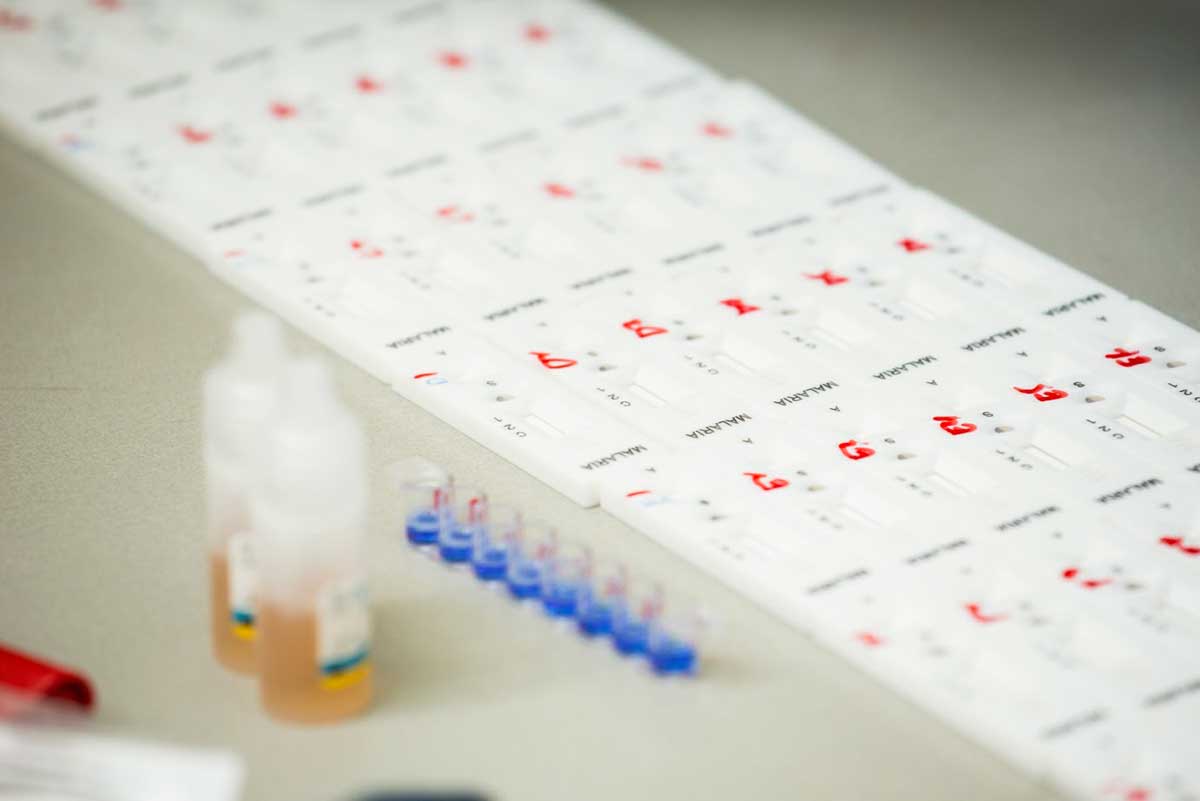
Rapid diagnostic tests (RDTs) are a key tool for routine diagnosis of malaria. These portable and disposable tests are simple to use and do not require laboratory infrastructure. However, the variable quality of RDTs on the market posed a challenge for countries in choosing which product to purchase.
Download the project evaluation
Our response
This project aimed to address quality control of RDTs at two different points in the supply chain: before products were procured, and through in-country evaluations at national referral labs.
The grant also aimed to make a quality-control system for RDT performance sustainable by speeding up the transition to recombinant proteins, catalyzing the market for recombinant-protein RDT quality control.
This project simplified and standardized quality control of RDTs, improved clinical efficiency and reduced testing costs. Lot-testing services for RDTs were decentralized to 12 national laboratories – 10 in Africa and two in Asia. The decentralized system used affordable and reproducible recombinant antigens to screen RDT lots instead of clinical samples, which are expensive to collect, characterize and maintain. The grant contributed to an improved quality of diagnostics.
The availability of affordable quality standards and practices promoted progress towards the World Health Organization’s (WHO) goal of universal access to diagnosis before malaria treatment. The program transitioned to WHO prequalification in January 2018.