Note: Unitaid is seeking projects that are led by local, South-based implementers – i.e. organizations that are headquartered in the sub-Saharan African country of implementation.
Under this call, Unitaid is soliciting proposals that will implement cost-effective and high impact approaches to introduce and scale lenacapavir for HIV prevention, in the context of broader PrEP options as relevant, to reach populations at high-risk in areas with high levels of transmission. In order to compare the cost-effectiveness and impact of innovative delivery approaches proposed under this call to the standard of care, selected countries are expected to have plans for lenacapavir rollout. Proposals must submit documentation of government support and intention to introduce lenacapavir in the near-term. While the geographic scope of this targeted call is early-adopter sub-Saharan African countries, Unitaid intends in the future to support adoption in additional regions. In addition, Unitaid’s ongoing grants are working already to enable broader access to lenacapavir both at market and country level, as described before (see section Context).
Proposals should seek to demonstrate impact, at (sub)national level within the project period. In other words, Unitaid is not seeking small pilots or demonstration projects, but rather implementation that will reach sufficient number of individuals at high risk of HIV acquisition to achieve measurable epidemic impact and help build the lenacapavir market. Proposals should justify the proposed scale to reach such objectives. Proposals should also clearly indicate and justify the number of individuals they intend to reach and the associated requirements for lenacapavir supply.
Community engagement is a critical pillar of Unitaid’s Strategy. Proposals must include strong and meaningful community engagement in all stages of project design and implementation. This is particularly relevant as this Call for Proposals seeks innovative strategies for the introduction and scale of lenacapavir that can complement facility-based approaches with other tailored and community-based approaches or other decentralised services.
As early-adopter countries advance on plans for lenacapavir national authorization and introduction, probably in early 2026, strong preference will be given to proposals that demonstrate readiness to implement in a similar timeframe. To facilitate rapid execution and country transition for sustainability, strong preference will be given to proposals that are led by local sub-Saharan African-based entities. Depending on the proposals received, Unitaid will also consider proposals with consortia including sub-Saharan African-based entities, as described in “Additional Considerations” and with extensive technical expertise in implementation of HIV PrEP services and programs and associated implementation research related to cost-effectiveness and impact.
Proposals that seek to achieve a scale required to demonstrate impact at national or subnational level within a single country are encouraged. Multi-country proposals will also be accepted; however careful consideration should be given to the readiness to implement quickly, the preference for a locally based lead implementer and the scale required for sustained impact (ie lenacapavir provided to a sufficient number of individuals at high risk of HIV acquisition to demonstrate measurable epidemic impact).
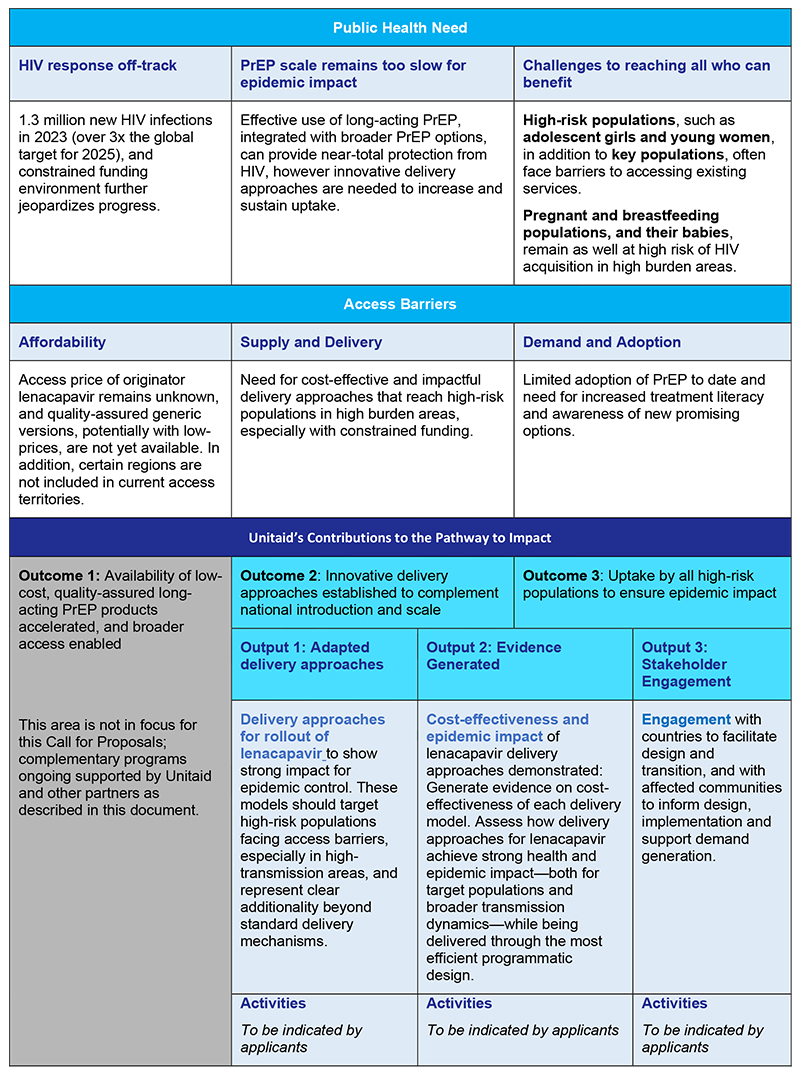
Proposals under this Call for Proposals are expected to contribute to the overarching objectives for lenacapavir (reflected in the Theory of Change shown below) by advancing Outcomes 2 and 3. Complementary investments (ongoing and planned, by Unitaid and partners) are addressing Outcome 1 (Accelerating availability of low-cost, quality assured generic products), as well as complementary work to support early adopter countries (Outcomes 2 and 3) , as described in the previous section.
Table 1: Unitaid’s Long-Acting PreP Portfolio-Level Theory of Change
[Click here to enlarge image]
Areas of work
Proposals should focus on the implementation and evidence generation of cost-effective and impactful approaches for the introduction to enable scaled up use of lenacapavir, with specific consideration to country priorities and community perspectives. These projects aim to be targeted and fast-paced, supporting national plans for lenacapavir rollout, complementing facility-based implementation with community-based and other decentralized and tailored services to the different target populations as most relevant for the local cycles of HIV transmission. Therefore, proposals should be very targeted and not seek to have several other areas of work beyond this scope.
Proposals should develop the following areas of work:
- Implementation of delivery approaches for rolling out lenacapavir that:
- Optimize the yield in terms of cost and impact.
- Reach large numbers of high-risk populations in areas with high levels of transmission that may face barriers to accessing existing HIV prevention services, justifying the scale proposed for the expected impact on the selected target group(s).
- Complement national plans for rollout of lenacapavir in sub-Saharan African countries.
- Demonstrate impact at national or subnational level within a project period of approximately three years.
- Consider effective tracking use of PrEP in the project’s target population, in consideration of national tracking systems.
- Integrate lenacapavir as part of a comprehensive PrEP program (e.g. alongside other tools such as oral PrEP) and potentially alongside other services for same target group.
- Support treatment literacy and demand generation activities tailored to the proposed delivery approaches and target populations.
- Reflect communities’ preferences and expertise and supports treatment literacy and demand generation.
- Evidence generation of:
- Cost-effectiveness to demonstrate how the delivery approach for lenacapavir for a given target population group minimizes programmatic costs to be absorbable by the country after project transition.
- Health impact on the specific target population group addressed by the project with the proposed delivery approach as compared to the impact of other existing delivery systems for PrEP services in the same population group.
- To generate this evidence, costing data will be collected during implementation, and impact will be modeled using existing context-specific data and assumptions.
- Stakeholder engagement with:
- Countries to ensure continual alignment, real-time sharing of learnings for country adoption, and transition of approaches to national programs, as well as informing other countries.
- Affected communities to ensure their meaningful input and expertise informs design, implementation and demand generation, and they support increased treatment & prevention literacy and awareness, as further described under Additional Considerations.
Additional information
Criteria
Proposals must be targeted, with clear, focused areas of work, as outlined in this Call for Proposals, that reach underserved high-risk populations in areas with a high incidence of HIV and complement countries’, Unitaid’s and other partners’ parallel plans.
Proponents must also meet the following criteria to be considered:
- Readiness: Demonstration of ability to begin implementation as soon as possible to accompany nationalintroduction of lenacapavir.
- Innovation: Implementation of innovative delivery approaches that complement facility-based rollout of lenacapavir with community-based and decentralized approaches. This may include leveraging existing platforms currently not in use or under-utilized for HIV PrEP services, supported by innovative demand generation approaches.
- Cost-effectiveness: Clear and feasible hypothesis for how the proposed delivery approaches for selected population and sub-geographies will be cost-effective, a proposed way to collect the required evidence and identification of drivers that can support countries accelerate and expand use of lenacapavir for those in need.
- Size: Seeks to achieve impact in the affected area within the project period (i.e. small pilot/demonstration projects are excluded), thereby requiring projects to have sufficient size to demonstrate the yield and impact of the selected approaches.
- Impact: Clear hypothesis on how the proposed delivery approaches for selected population and sub-geographies will be conducive of decreased HIV transmission and proposed ways to measure it.
- Community engagement: Strong and meaningful community engagement in all stages of project governance, design and implementation.
- Geographic scope: Proposals must be focused on sub-Saharan African countries that plan to introduce lenacapavir as part of national programs as soon as market authorization is granted, with consideration of sub-geographies with highest risk.
- Government support: Proposals must submit letters of support from government stakeholders that confirm the country’s intention to introduce lenacapavir in the near-term, with clear justification in case such a supporting letter is not available.
- Experience: Proposals must be led by proponents with extensive technical expertise in the implementation of HIV PrEP services and programs, including integration of services with other health programs, and demonstrated experience in associated implementation research. Community-based organizations participating in the project should also have demonstrated experience in the services required.
In addition, please note the following criteria are not required but will strengthen proposals:
- Protocol for cost-effectiveness and impact studies: Proponents are encouraged to share draft protocols, if available, for cost-effectiveness and impact studies. Submitted protocols will be reviewed only by external experts, under confidentiality, to assess alignment with the objectives of this Call for Proposals; no recommendations or review will be performed on such protocols. Submitting protocols at this stage is not a requirement but will help indicate readiness to implement rapidly.
- Co-funding: Proponents are encouraged to indicate potential, or secured, sources of co-funding for the program or for complementary activities.
Expectations for deliverables
Proposals under this Call for Proposals are expected to outline concrete deliverables that will be produced throughout the project period and should include a cost-effectiveness and impact study.
Impact we are seeking
Through this Call for Proposals, Unitaid aims to implement catalytic interventions that ensure lenacapavir potential is realized by all in need, substantially reducing HIV transmission within the target population and geographical area and beyond by contributing to an impactful global scale up of lenacapavir. In addition, by unlocking the use of lenacapavir by additional target groups in heavily affected areas, it is expected to help build the market for lenacapavir.
Additional considerations
Unitaid considers working with affected communities, including grassroots groups, and civil society organizations, to be critical and strongly encourages adopting inclusive approaches. This includes considering and adequately funding and resourcing their meaningful and continued engagement and participation in decision-making throughout the design, planning, implementation, and evaluation of activities.
Unitaid is primarily looking for proposals from south-based lead implementers (i.e. organizations that are headquartered in a low- or middle-income country) with experience in leading the implementation of large-scale projects that support access to health products in low- and middle-income countries. We also will consider the proposals covering the meaningful inclusion of south-based sub-implementers, where feasible and relevant, in proposed project implementation consortia. In such cases, when adjudicating proposals, Unitaid will consider the budget proportion allocated to global south partners, recommending a minimum of 50%. However, Unitaid’s objective of progressively retaining an increased number of lead implementing partners from the global south does not preclude proposals that are led by or including partners from the global north, where their role is deemed complementary and important for the success of the proposal.
Proposals should be carefully targeted, reflecting focused interventions to implement cost-effective and impactful delivery approaches for high-risk populations in areas with high levels of transmission. Proposals should clearly indicate the level of effort and budget for each activity. Given the current uncertainties about the supply arrangements and access price for lenacapavir for low- and middle-income countries, and as indicated in the proposal template, the budget for procurement of lenacapavir should be kept separate from the rest of the project budget and will be considered a placeholder.
Proposals should demonstrate value for money and measurable impact. Proposals should also include analysis of pathways to impact, scalability, and sustainability of key interventions.
Areas out of scope for this Call include clinical trials, small-scale pilots/demonstration projects, and service delivery that does not measure the cost-effectiveness and impact of innovative delivery approaches in sub-Saharan African countries that are introducing lenacapavir as part of national programs.

