The problem
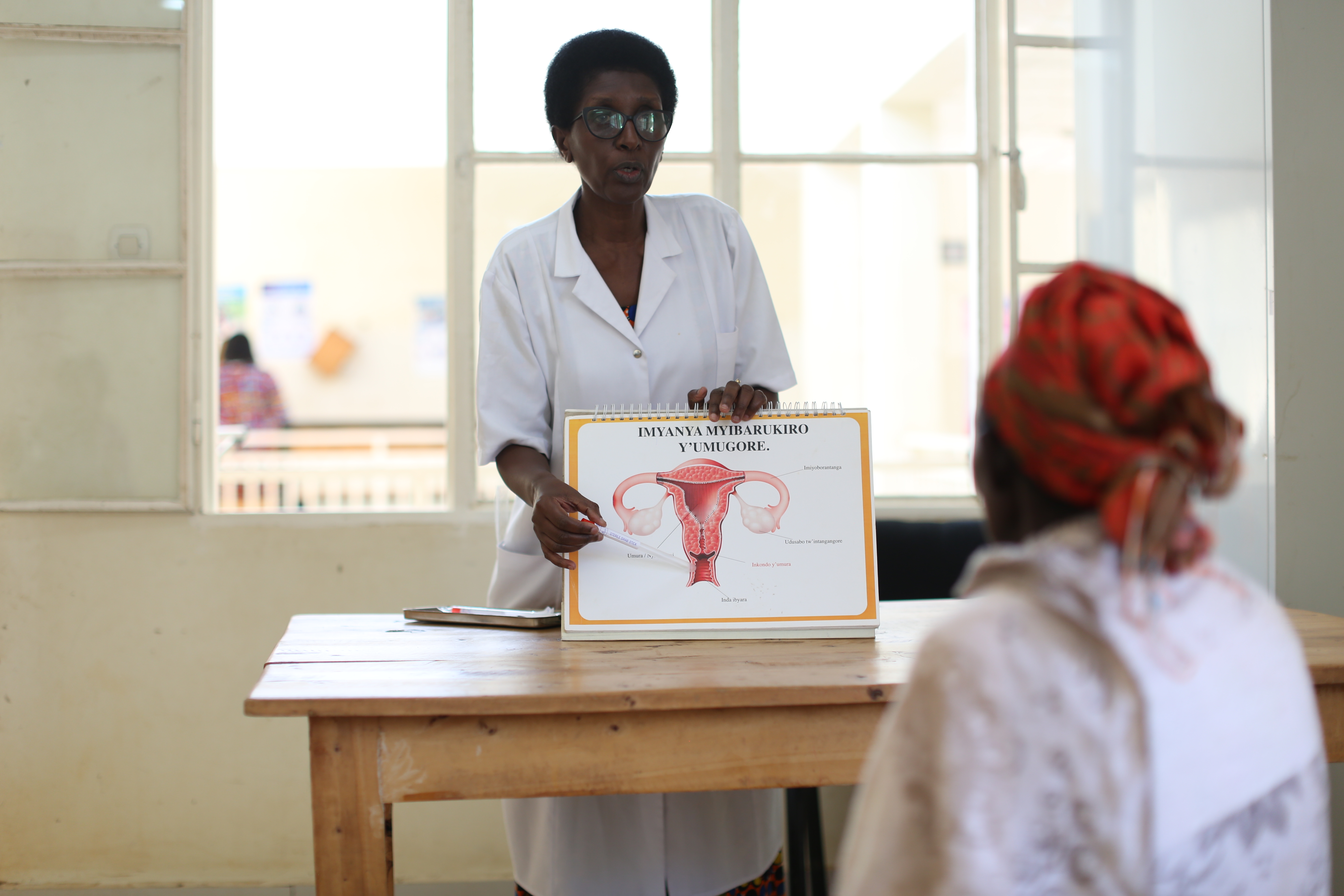
Despite progress in reducing vertical transmission of HIV from mother to baby at birth, many infants still become infected with HIV. Without treatment, HIV progresses rapidly in infants and could cause them to die within several months. When this project began, infant diagnosis required sending samples off to central laboratories, a process that could take months, critically delaying a child’s access to life-saving treatment. If a child did get diagnosed, HIV medicines were not available in child-friendly formulations, which severely hampered treatment success.
Download the project evaluation
Progress
Innovative technologies that made quick and decentralized infant diagnosis possible proved to be a game-changer, enabling, in many cases, same-day diagnosis and connection to treatment – compared to an average of three months previously. The project integrated point-of-care early infant diagnosis into health systems and facilities in nine countries, created tools for scale up, demonstrated public health impact and cost-effectiveness of the tools, negotiated lower prices and more sustainable pricing models, improved service and maintenance conditions and secured funding commitments from national governments and international donors towards scale-up.
The project then transitioned to focus on securing access to improved child-friendly drug formulations for HIV medicines, to ensure that once a child got a diagnosis, they could access the highest-quality treatment for the best health outcomes.
This project was part of a larger investment from Unitaid that aimed to reduce delays in infant diagnosis and improve access to care. Together, the two projects are estimated to have prevented 8,100 infant deaths through rapid HIV diagnostics and generated US$5.9 million in savings to health systems by advancing more cost-effective testing methods.