The problem
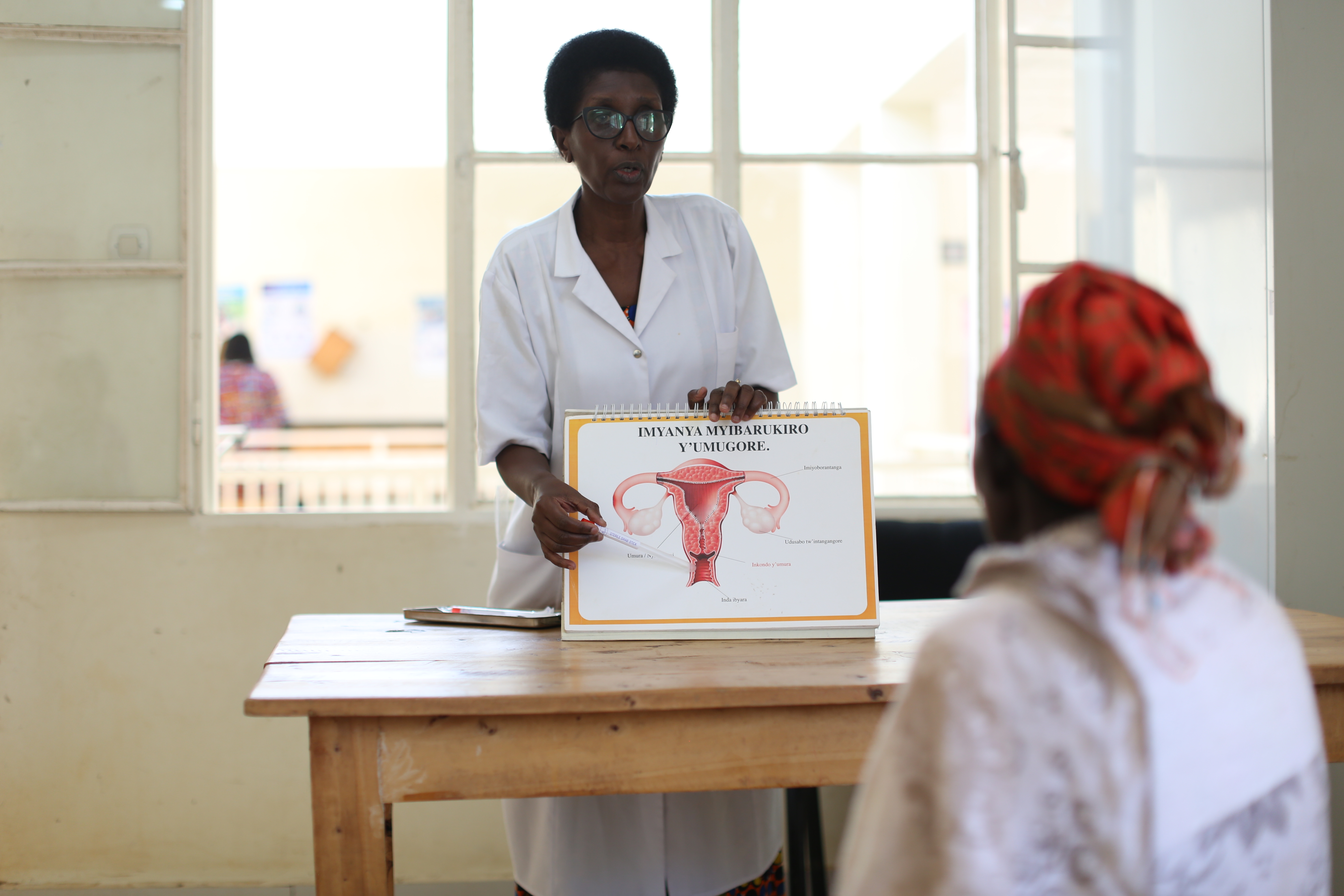
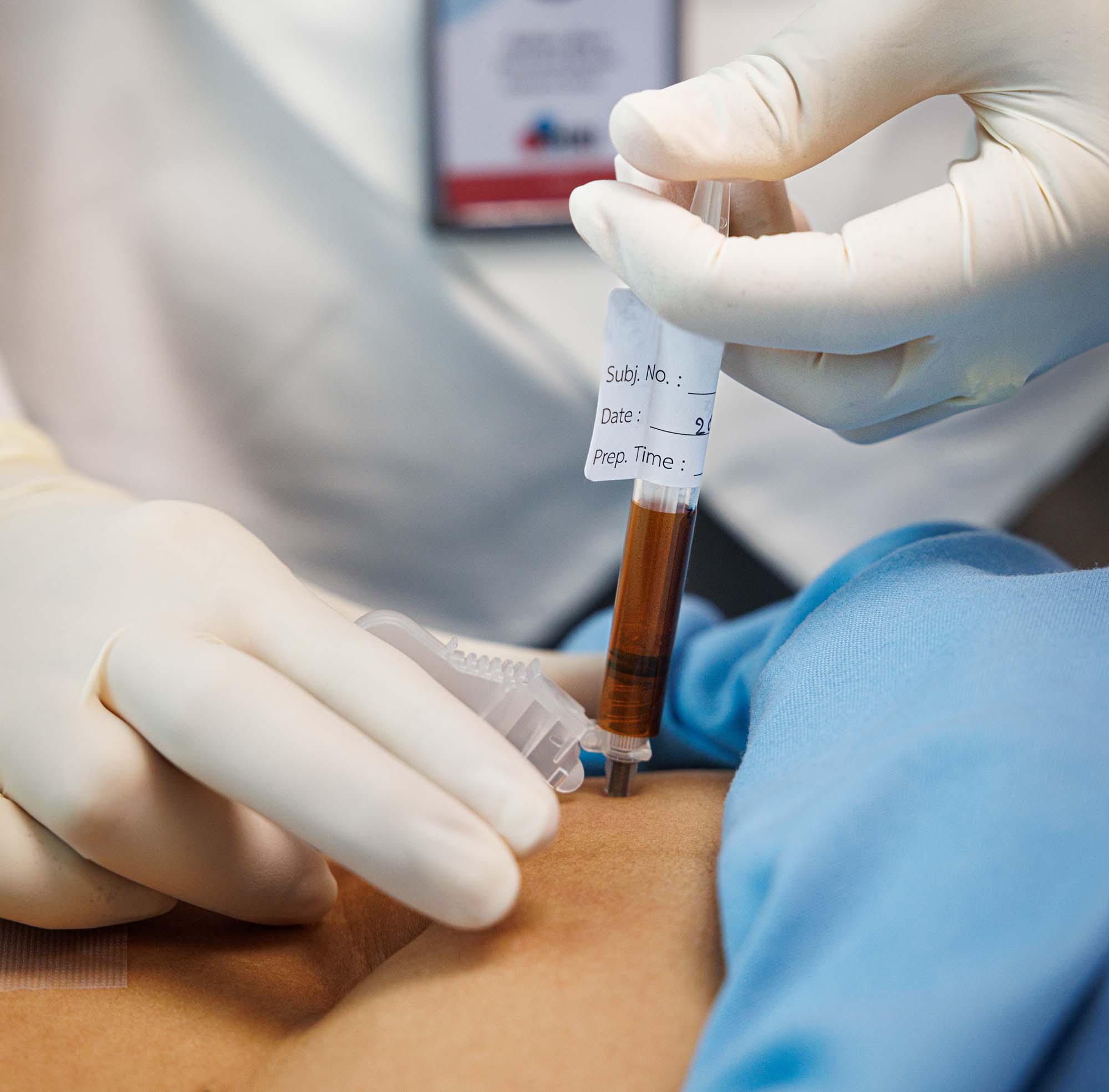
Diagnosing HIV in infants used to require samples to be sent to central laboratories for testing, a process that often took months. Infants are particularly vulnerable to HIV infection and, without a diagnosis to enable them to access treatment, they could become severely ill and even die while awaiting test results. At the project’s outset, only about half of all infants exposed to HIV were tested and, because of logistical challenges with centralized testing, only half of those tested received their results. Viral load monitoring, which is critical to ensuring that treatment is working effectively for all people on HIV treatment, had similarly long wait times. This affected the timeliness of treatment-related decisions, particularly critical for people with failing treatment or pregnant women.
Download the project evaluation
Progress
Innovative devices offered the possibility to make test results available more quickly and at lower levels of health systems. Together with a complementary Unitaid-funded initiative, the project worked to bring these vital new tools to market, increase demand and build capacity to enable newborns to get tested quickly, at the point of care, often with same day results, compared to an average of three months previously required. Working across 11 countries, this project played a critical role in proving the feasibility and cost-effectiveness of point-of-care testing for early infant diagnosis and viral load monitoring.
This project was part of a larger investment from Unitaid that aimed to reduce delays in infant diagnosis and improve outcomes. Together, the two projects are estimated to have prevented 10,000 infant deaths through HIV diagnosis and viral load monitoring and generated US$11.3 million in savings to health systems by averting opportunistic infections, reducing HIV transmission and advancing more cost-effective testing methods.